Advances In Electrode Engineering: Pioneering Materials, Architectures, And Interfaces For Next-generation Electrochemical Devices
Electrode engineering stands as a cornerstone of modern electrochemistry, underpinning the performance, efficiency, and longevity of a vast array of technologies, from energy storage systems to biosensors and electrocatalytic converters. Recent research has moved beyond simple material selection, evolving into a sophisticated discipline that meticulously designs electrodes from the atomic scale to the macroscopic structure. This article delves into the latest breakthroughs in materials synthesis, structural design, and interface manipulation, highlighting how these advances are propelling electrochemical devices toward new frontiers of capability.
Novel Material Discovery and Nanoscale Design
The quest for higher capacity, faster kinetics, and improved stability begins at the material level. While graphite anodes and lithium transition metal oxide cathodes remain workhorses for lithium-ion batteries (LIBs), engineers are pushing boundaries with innovative alternatives. For instance, silicon (Si) anodes, with a theoretical capacity nearly ten times that of graphite, have long been plagued by massive volume expansion during lithiation. Recent breakthroughs involve sophisticated nanostructuring to mitigate this issue. The development of porous Si nanoparticles, yolk-shell structures (where the active Si core has room to expand within a protective carbon shell), and conformally coated Si nanowires have dramatically improved cyclability. As noted by Liu et al. (2022), such engineered nanostructures effectively accommodate mechanical strain, preventing pulverization and maintaining electrical contact.
Similarly, on the cathode side, there is a significant push towards nickel-rich layered oxides (NMC/NCA) and lithium-rich manganese-based materials to increase energy density. Engineering these materials involves precise elemental doping and surface coating to suppress detrimental phase transitions and interfacial side reactions. For example, a nanoscale lithium phosphate coating on NMC811 particles has been shown to significantly enhance thermal stability and cycle life (Qiu et al., 2021).
Beyond LIBs, electrode engineering is critical for emerging technologies. In electrocatalysis, for the hydrogen evolution reaction (HER) or oxygen reduction reaction (ORR), engineering single-atom catalysts (SACs) anchored on nitrogen-doped graphene represents a pinnacle of material design. This architecture maximizes the utilization of every precious metal atom (e.g., Pt, Ir) and tailors the electronic environment for optimal catalytic activity (Zhang et al., 2020).
Revolutionizing Electrode Architecture in 3D
A paradigm shift from traditional 2D planar electrodes to bespoke 3D architectures is unlocking unprecedented performance metrics. This involves designing the macroporous scaffold of the electrode itself. 3D printing, or additive manufacturing, has emerged as a powerful tool to create electrodes with digitally controlled, hierarchical pore networks. These structures facilitate rapid ion transport by shortening diffusion pathways and ensuring efficient electrolyte penetration, which is crucial for high-power applications.
The integration of graphene aerogels, carbon nanotubes (CNTs), and other self-assembled frameworks creates lightweight, highly conductive, and mechanically robust 3D current collectors. Replacing heavy, inert aluminum or copper foils with these freestanding architectures increases the device's specific energy (energy per unit weight). A recent study demonstrated a 3D-printed graphene aerogel electrode with a triply periodic minimal surface structure, which exhibited exceptional rate capability and stability in supercapacitors due to its optimal flow of electrons and ions (Zhu et al., 2021).
Furthermore, the concept of "gradient electrodes" is gaining traction. Here, the composition or porosity is intentionally varied throughout the electrode's thickness. For instance, an electrode may have a high-density, high-capacity active material layer close to the current collector for energy, and a more porous, conductive additive-rich layer near the separator for power. This zoning addresses the conflicting demands of energy and power density within a single electrode.
Mastering the Electrode-Electrolyte Interface
The performance and degradation of electrochemical devices are predominantly governed by the complex processes occurring at the electrode-electrolyte interface. Therefore, interfacial engineering is arguably the most critical aspect of modern electrode design. A primary focus is on the formation of stable, protective interphases.
In batteries, the Solid Electrolyte Interphase (SEI) on the anode and the Cathode Electrolyte Interphase (CEI) on the cathode are essential yet fragile. Unstable SEI growth consumes active lithium and electrolytes, leading to capacity fade. Advanced engineering strategies now aim topre-forman artificial, robust SEI layer. This can be achieved through electrolyte additives—such as fluoroethylene carbonate (FEC)—that polymerize to form a protective film, or through pre-treatment methods that electrochemically deposit a tailored interface before device operation. Similarly, artificial CEI layers made of Al2O3, Li3PO4, or polymers deposited via atomic layer deposition (ALD) or molecular layer deposition (MLD) provide a physical barrier that shields the cathode from electrolyte decomposition, especially at high voltages (Cao et al., 2022).
For aqueous systems like supercapacitors and certain batteries, engineering the electrical double layer (EDL) is key. Using ionic liquids or concentrated "water-in-salt" electrolytes can significantly widen the electrochemical stability window, allowing for higher operating voltages and energies. The electrode surface chemistry can also be modified to promote specific ion ordering within the EDL, enhancing charge storage capacity.
Future Outlook and Challenges
The future of electrode engineering lies in increasing sophistication and the integration of multi-functional design. We are moving into an era of "smart" electrodes that can self-heal, sense their state of health, or even adapt their properties in response to operational demands. The use of machine learning and AI will accelerate the discovery of new material combinations and optimal 3D architectures by navigating vast design spaces that are intractable for humans alone.
Scaling these advanced manufacturing techniques, such as ALD for interface coating or 3D printing for structural control, to industrial levels remains a significant economic and technical hurdle. Furthermore, sustainability must be woven into the fabric of electrode design. Future research must prioritize the use of abundant, non-toxic materials and develop efficient recycling pathways for these complex, multi-material structures.
In conclusion, electrode engineering has transformed from a secondary consideration into a primary driver of innovation in electrochemical technology. By concurrently manipulating materials, architecture, and interfaces, researchers are creating electrodes that are no longer passive components but highly engineered systems, paving the way for the next generation of high-performance, durable, and sustainable energy storage and conversion devices.
ReferencesCao, X., et al. (2022). Artificial Interphases for Highly Stable Lithium Metal Anodes.Chemical Reviews, 122(11), 10970-11021.Liu, Y., et al. (2022). Recent Progress on Silicon-Based Anodes for Lithium-Ion Batteries.Advanced Energy Materials, 12(5), 2101465.Qiu, L., et al. (2021). Surface Modification of Ni-Rich Cathode Materials for Lithium-Ion Batteries.Joule, 5(10), 2583-2604.Zhang, L., et al. (2020). Single-Atom Catalysts: Emerging Multifunctional Materials in Heterogeneous Catalysis.Advanced Energy Materials, 10(29), 2000759.Zhu, C., et al. (2021). 3D Printed Functional Graphene Networks for Supercapacitors.Nature Communications, 12, 3320.
Customized/OEM/ODM Service
HomSolar Supports Lifepo4 battery pack customization/OEM/ODM service, welcome to contact us and tell us your needs.


HomSolar: Your One-stop LiFePO4 Battery Pack & ESS Solution Manufacturer
Our line of LiFePO4 (LFP) batteries offer a solution to demanding applications that require a lighter weight, longer life, and higher capacity battery. Features include advanced battery management systems (BMS), Bluetooth® communication and active intelligent monitoring.

Customised Lithium Iron Phosphate Battery Casing
ABS plastic housing, aluminium housing, stainless steel housing and iron housing are available, and can also be designed and customised according to your needs.

HomSolar Smart BMS
Intelligent Battery Management System for HomSolar Energy Storage System. Bluetooth, temperature sensor, LCD display, CAN interface, UART interface also available.

Terminals & Plugs Can Be Customized
A wide range of terminals and plugs can be customised to suit the application needs of your battery products.

Well-designed Solutions for Energy Storage Systems
We will design the perfect energy storage system solution according to your needs, so that you can easily solve the specific industry applications of battery products.

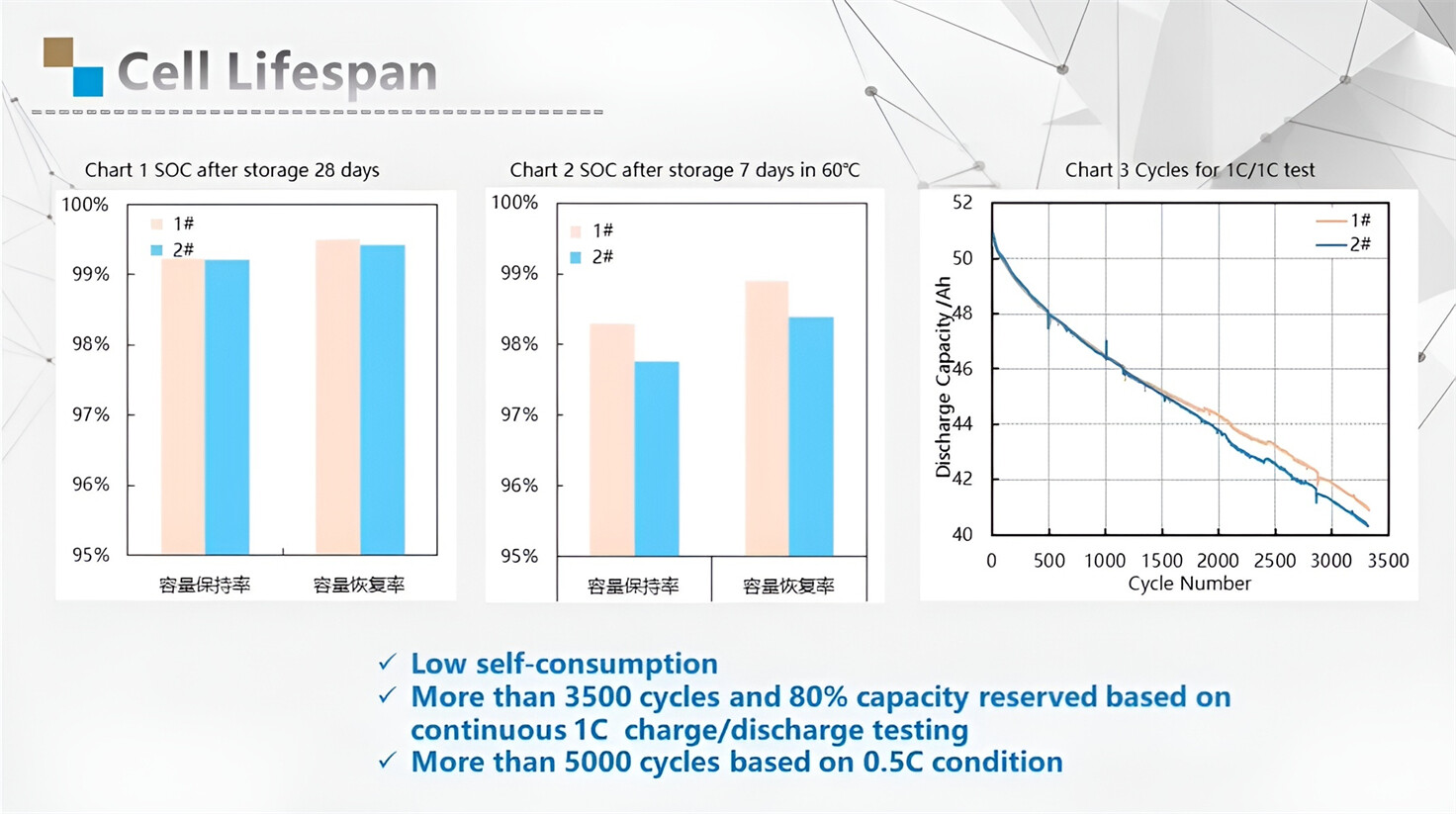
About Our Battery Cells
Our energy storage system products use brand new grade A LiFePO4 cells with a battery lifespan of more than 4,000 charge/discharge cycles.

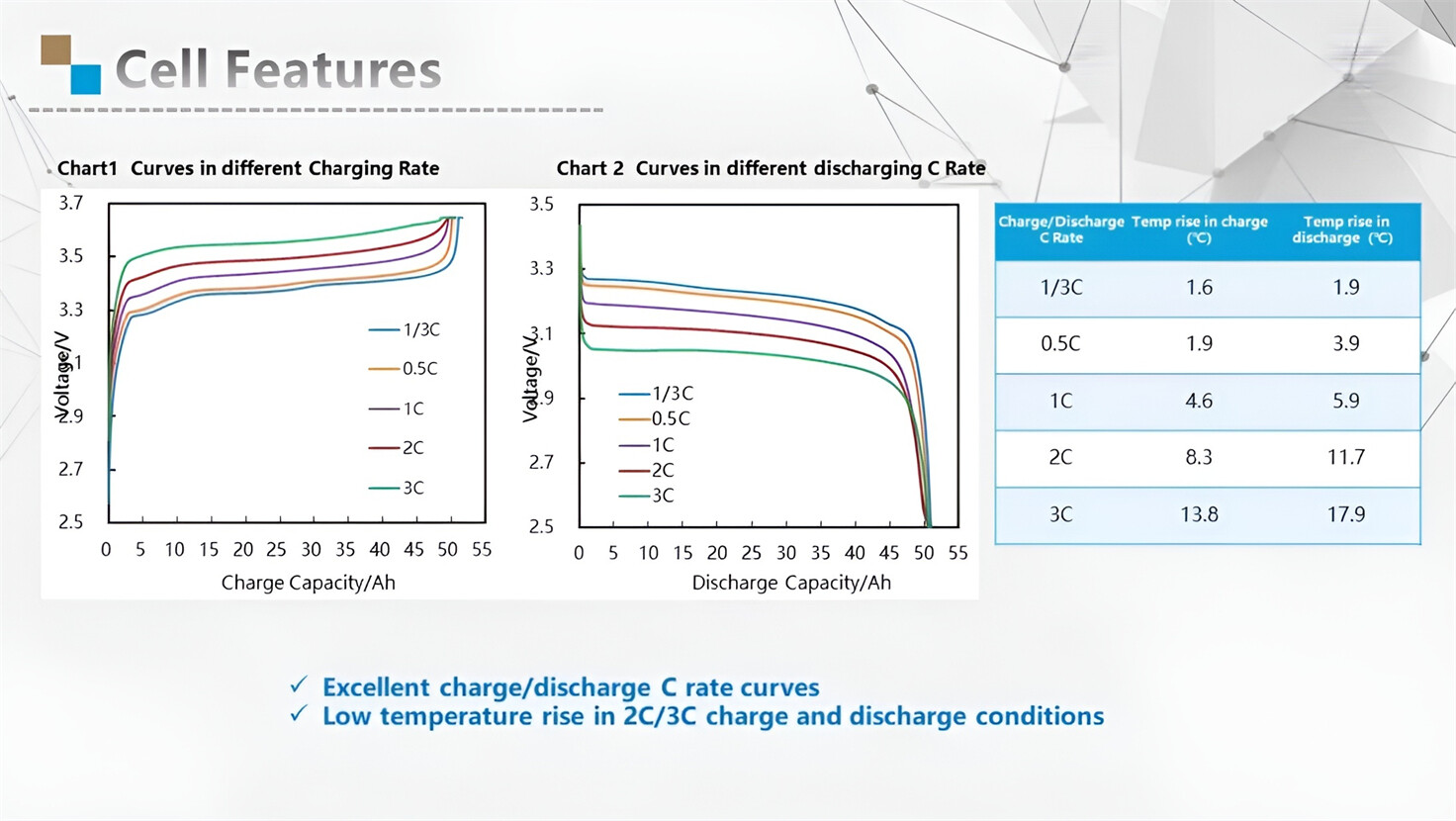
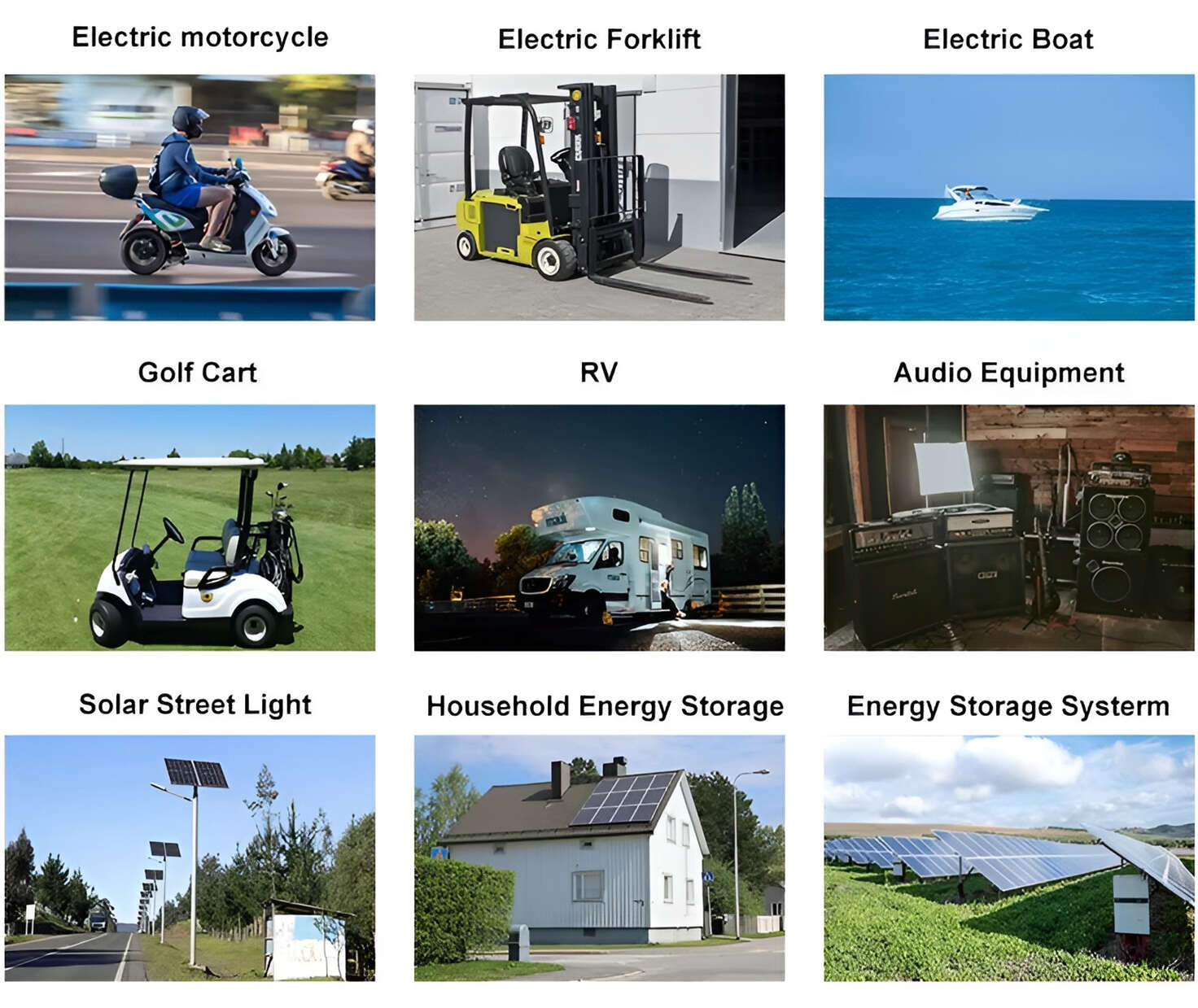
Applications in Different Industries
We supply customized & OEM battery pack, assemble cells with wiring, fuse and plastic cover, all the cell wires connected to PCB plug or built BMS.
Applications: E-bike, Electric Scooter, Golf Carts, RV, Electric Wheelchair, Electric Tools, Robot Cleaner, Robot Sweeper, Solar Energy Storage System, Emergency Light, Solar Power Light, Medical Equipment, UPS Backup Power Supply.
We can provide you with customized services. We have the ability to provide a vertical supply chain, from single cells to pack/module and to a complete power solution with BMS, etc.

HomSolar (Shenzhen) Technology Co., Ltd