Advances In Cation Doping: Engineering Next-generation Functional Materials
The strategic introduction of foreign cationic species into host crystal lattices, a process known as cation doping, has long been a cornerstone of materials science. Moving far beyond the simple charge compensation strategies of the past, recent research has elevated cation doping to a sophisticated tool for precisely engineering the electronic, optical, magnetic, and catalytic properties of a vast array of materials. This progress is driven by advanced synthesis techniques, high-resolution characterization, and computational modeling, enabling an unprecedented level of control over material behavior for applications ranging from energy conversion and storage to quantum computing.
Recent Research and Technological Breakthroughs
A significant frontier in cation doping is its application in stabilizing unconventional phases and enhancing the performance of energy materials. In the field of lithium-ion batteries, high-nickel layered oxide cathodes (e.g., LiNixMnyCo1-x-yO2, NMC) suffer from structural degradation and interfacial instability. Recent breakthroughs involve multi-element co-doping strategies. For instance, the co-doping of cations like Al3+ and Ti4+ has been shown to synergistically strengthen the crystal structure. Al3+ provides strong Al-O bonds that suppress oxygen loss and layer collapse, while Ti4+ inhabits the transition metal layer, inhibiting detrimental phase transitions and improving Li+ diffusion kinetics. A study by Qian et al. demonstrated that such a dual-doping approach in LiNi0.8Mn0.1Co0.1O2 significantly improved capacity retention after hundreds of cycles, paving the way for more durable and high-energy-density batteries [1].
Similarly, in perovskite solar cells (PSCs), cation doping is pivotal in addressing intrinsic instability issues. While pure lead-based perovskites are efficient, they are prone to ion migration and thermal degradation. The partial substitution of Pb2+ with smaller isovalent cations like Sn2+ or heterovalent cations like Sb3+ has yielded remarkable results. Doping with Sb3+ serves a dual purpose: it passivates ionic defects and induces a slight lattice strain that enhances charge carrier lifetime. Furthermore, the introduction of alkali metal cations (e.g., Rb+, Cs+) into the perovskite lattice has been shown to suppress halide segregation and improve phase stability, leading to devices that maintain high efficiency under operational stressors [2]. This precise "cation engineering" is key to commercializing PSC technology.
Beyond energy applications, cation doping is revolutionizing the field of two-dimensional (2D) materials and quantum materials. In transition metal dichalcogenides (TMDs) like MoS2, doping with Re or Nb atoms can transform the material's electronic character from semiconducting to metallic or even superconducting. A groundbreaking study demonstrated that controlled vanadium doping in WSe2 creates stable single-photon emitters, which are crucial components for quantum information processing [3]. The dopant atoms create localized electronic states that can be optically addressed, opening a pathway for designing quantum bits (qubits) in solid-state systems.
The synthesis and characterization techniques themselves have seen profound advancements. Traditional solid-state reactions often lead to inhomogeneous dopant distribution. Modern methods, such as flame spray pyrolysis and advanced chemical vapor deposition (CVD), allow for atomic-level precision in dopant incorporation. For example, pulsed laser deposition (PLD) enables the creation of oxide thin films with precisely controlled doping profiles, which is essential for developing novel oxide electronics. Coupled with these synthesis tools, state-of-the-art characterization techniques like aberration-corrected scanning transmission electron microscopy (STEM) and electron energy loss spectroscopy (EELS) can now directly image individual dopant atoms and map their local chemical environment, providing invaluable feedback for refining doping protocols [4].
Computational materials science has become an indispensable partner to experimentation. High-throughput density functional theory (DFT) calculations can screen thousands of potential host-dopant combinations to predict formation energies, optimal doping concentrations, and the resulting electronic structure modifications before any synthesis is attempted. This "materials-by-design" approach dramatically accelerates the discovery of new doped materials with tailored properties.
Future Outlook and Challenges
The future of cation doping research is exceptionally promising but also presents significant challenges. One major frontier is the move towards dynamic and spatially controlled doping. Researchers are exploring "active doping" strategies where an external stimulus, such as an electric field, light, or ionic liquid gating, can reversibly modulate the dopant concentration or valence state. This could lead to the development of novel reconfigurable electronics and neuromorphic computing devices whose fundamental properties can be altered on-demand.
Another critical direction is achieving absolute precision in single-atom doping. While progress has been made, placing a single dopant atom at a specific lattice site with 100% yield remains a formidable challenge. Advances in atomically precise fabrication techniques, such as scanning probe manipulation, may one day enable the construction of custom-designed quantum materials atom-by-atom.
For complex multi-element doping, understanding and controlling the interactions between different dopants is crucial. Synergistic and antagonistic effects are often poorly understood. Future research will need to leverage machine learning algorithms to decipher the complex, multi-variable relationships between synthesis parameters, dopant combinations, and final material properties. This will allow for the optimization of "cocktail" doping recipes for specific applications.
Furthermore, the stability of doped structures, especially under harsh operating conditions, requires continued investigation. In catalysts or battery electrodes, dopant segregation or dissolution can lead to performance decay. Developingin-situandoperandocharacterization methods to observe these degradation mechanisms in real-time will be essential for designing more robust materials.
Finally, the exploration of cation doping should expand further into emerging material classes, such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and high-entropy oxides (HEOs). Doping in these complex structures could unlock unprecedented catalytic activity, selective gas separation capabilities, or novel magnetic phenomena.
In conclusion, cation doping has evolved from a simple compositional modification into a powerful and versatile paradigm for materials design. The convergence of sophisticated synthesis, cutting-edge characterization, and predictive computational power is enabling a new era of functional materials. As we learn to command the dopant with ever-greater precision, we unlock the potential to tailor-make the materials that will power the next generation of technological breakthroughs.
References
[1] Qian, G., et al. "Dual-element doping strategy to stabilize LiNi0.8Mn0.1Co0.1O2 cathode materials: A combined study of first-principles calculations and experimental validation."Advanced Energy Materials, vol. 12, no. 15, 2022, 2103681.
[2] Zhang, T., et al. "Cation engineering in lead halide perovskites: From structural stability to performance reproducibility."Joule, vol. 5, no. 8, 2021, pp. 2122-2137.
[3] Chakraborty, C., et al. "Site-selectively generated photon emitters in monolayer WSe2 via local potentiometry."Nature Materials, vol. 18, no. 9, 2019, pp. 963-969.
[4] Hwang, J., et al. "Direct visualization of dopant atom distribution in Y-doped BaZrO3 proton conducting electrolyte."Nano Letters, vol. 20, no. 5, 2020, pp. 3811-3817.
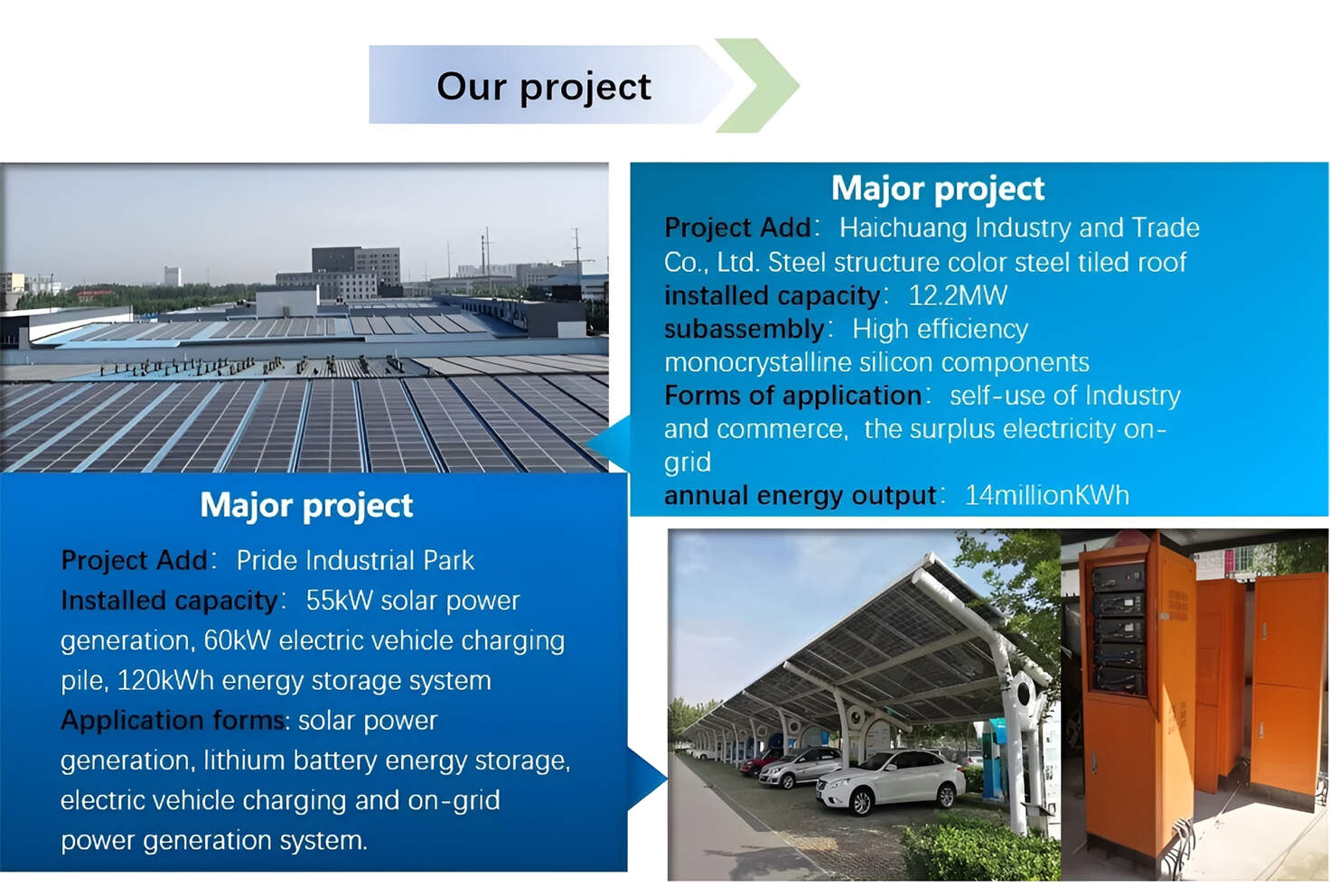
Customized/OEM/ODM Service
HomSolar Supports Lifepo4 battery pack customization/OEM/ODM service, welcome to contact us and tell us your needs.


HomSolar: Your One-stop LiFePO4 Battery Pack & ESS Solution Manufacturer
Our line of LiFePO4 (LFP) batteries offer a solution to demanding applications that require a lighter weight, longer life, and higher capacity battery. Features include advanced battery management systems (BMS), Bluetooth® communication and active intelligent monitoring.

Customised Lithium Iron Phosphate Battery Casing
ABS plastic housing, aluminium housing, stainless steel housing and iron housing are available, and can also be designed and customised according to your needs.

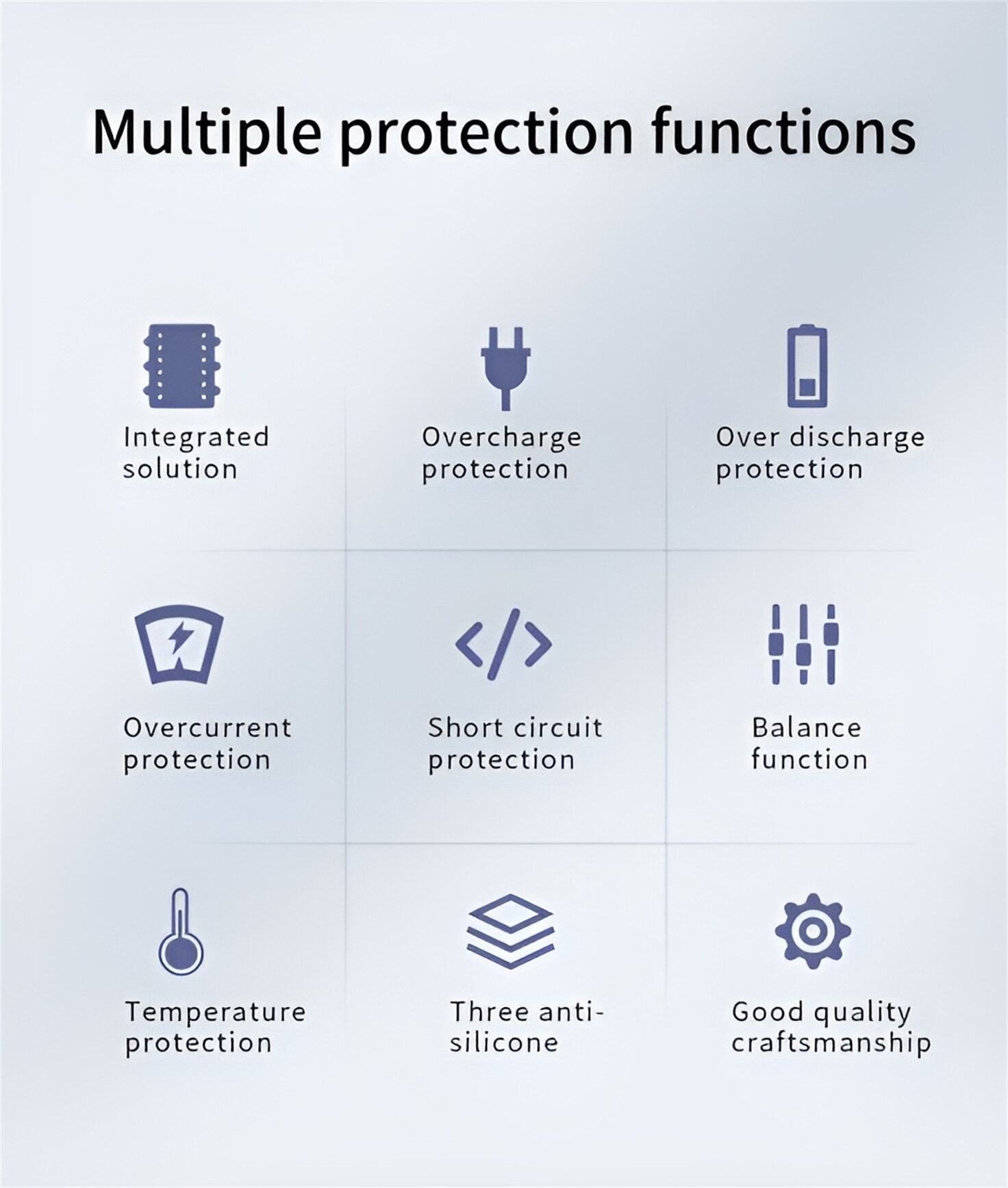
HomSolar Smart BMS
Intelligent Battery Management System for HomSolar Energy Storage System. Bluetooth, temperature sensor, LCD display, CAN interface, UART interface also available.

Terminals & Plugs Can Be Customized
A wide range of terminals and plugs can be customised to suit the application needs of your battery products.

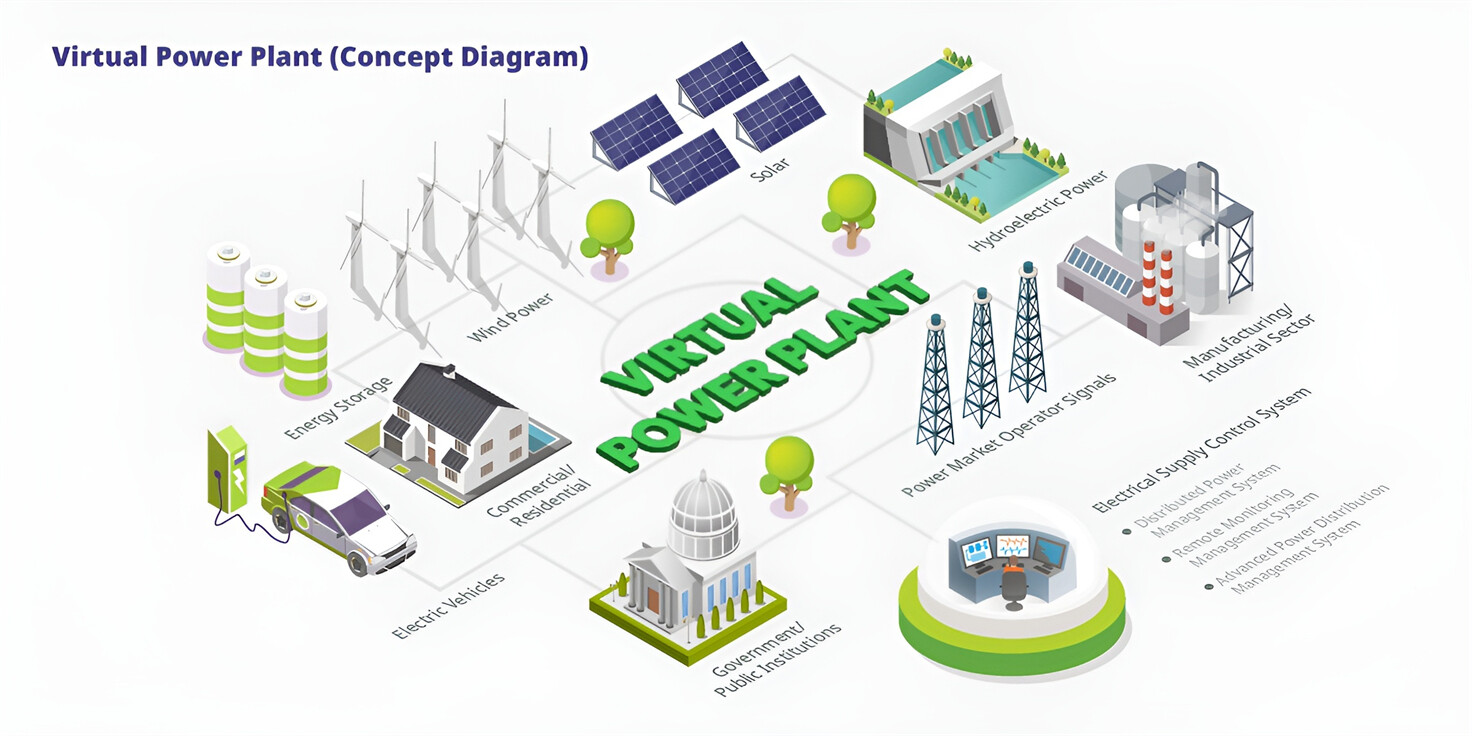
Well-designed Solutions for Energy Storage Systems
We will design the perfect energy storage system solution according to your needs, so that you can easily solve the specific industry applications of battery products.

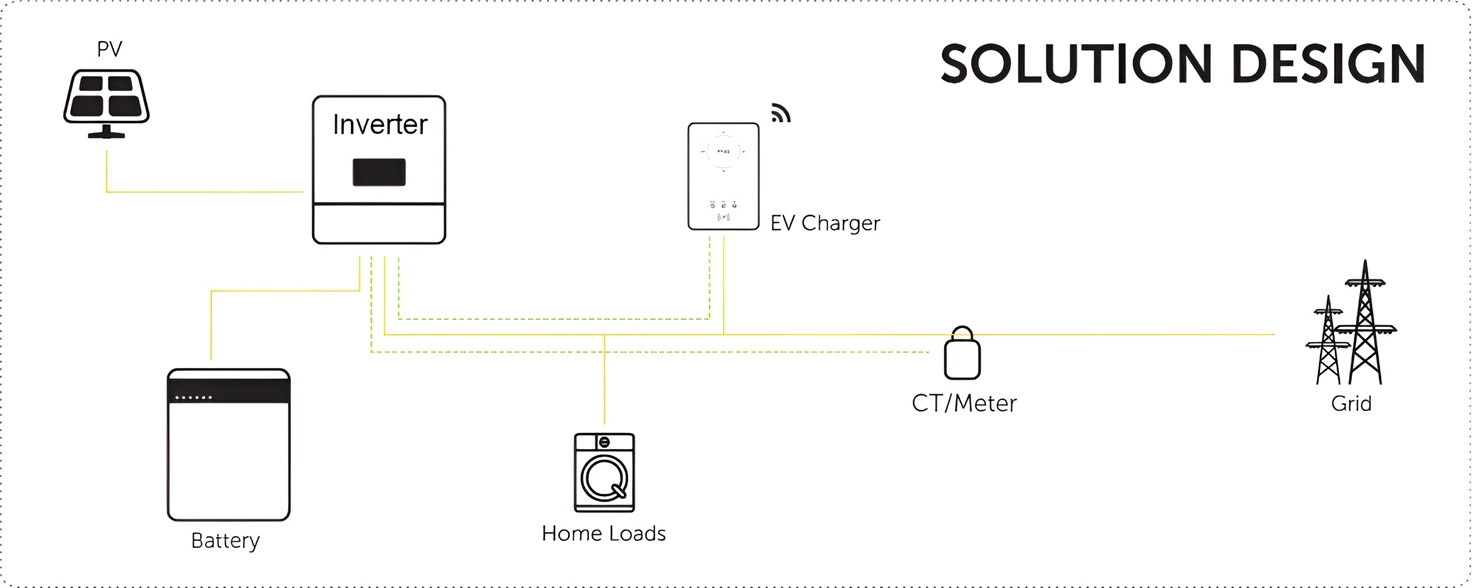
About Our Battery Cells
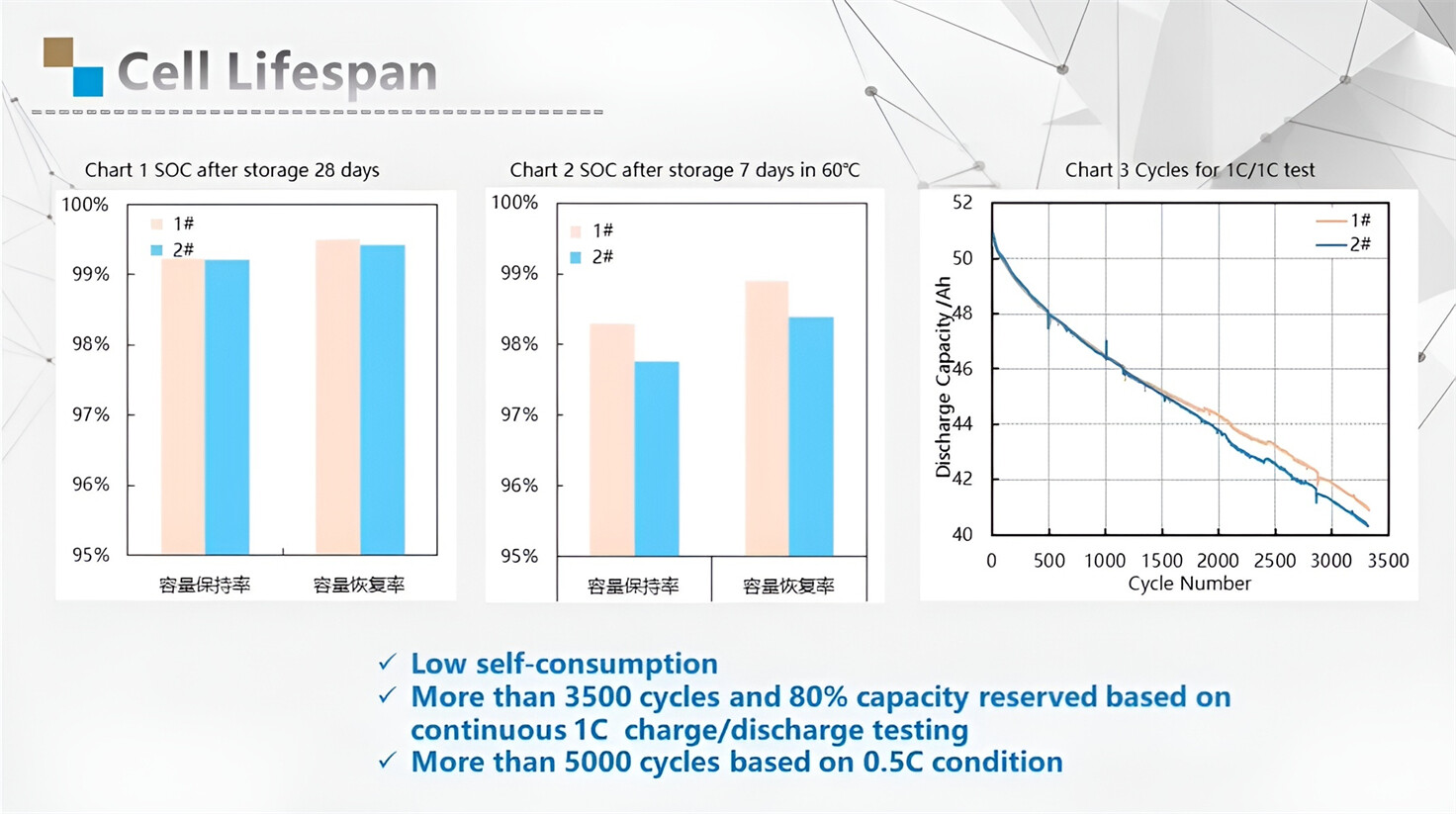
Our energy storage system products use brand new grade A LiFePO4 cells with a battery lifespan of more than 4,000 charge/discharge cycles.

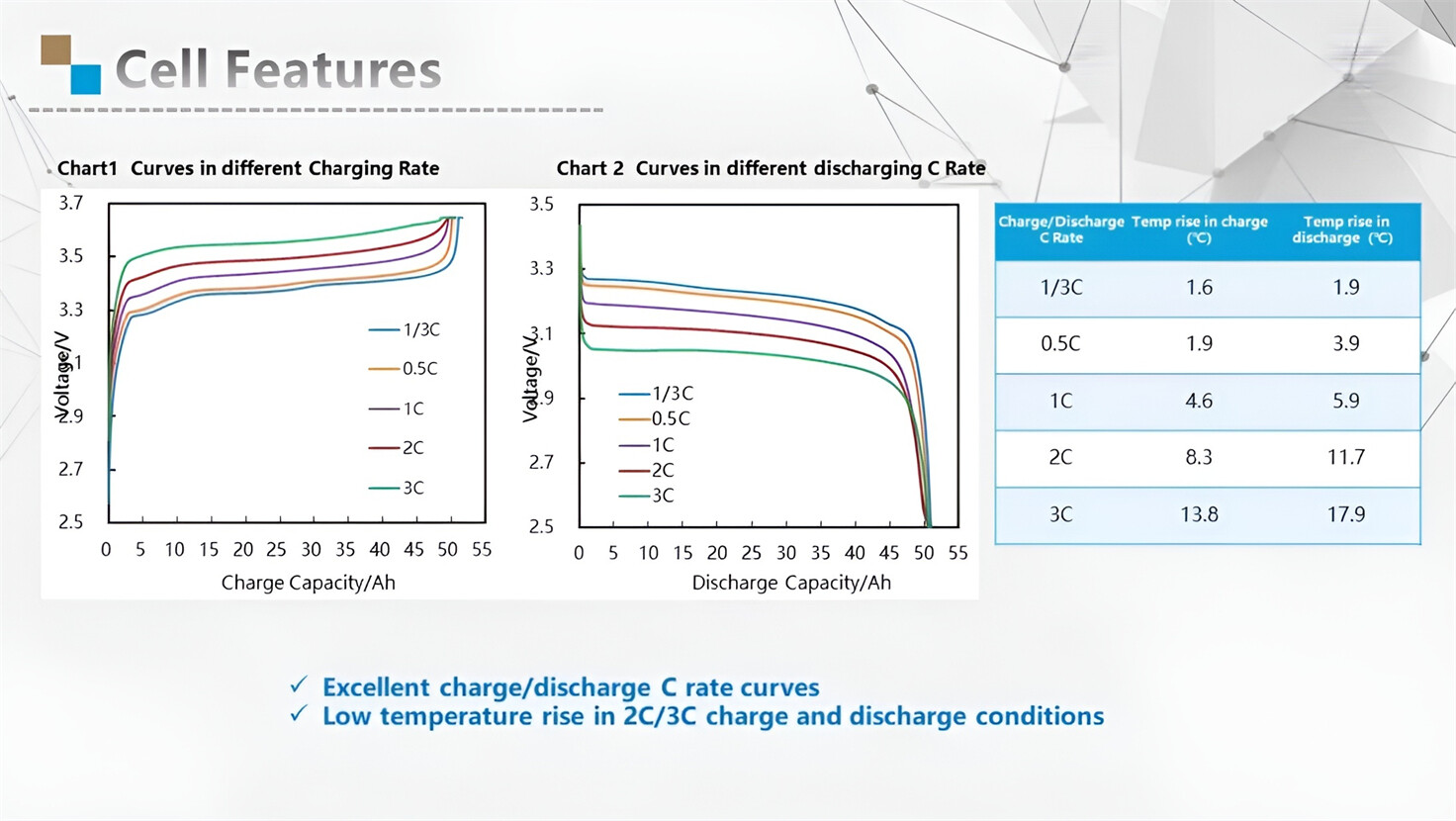
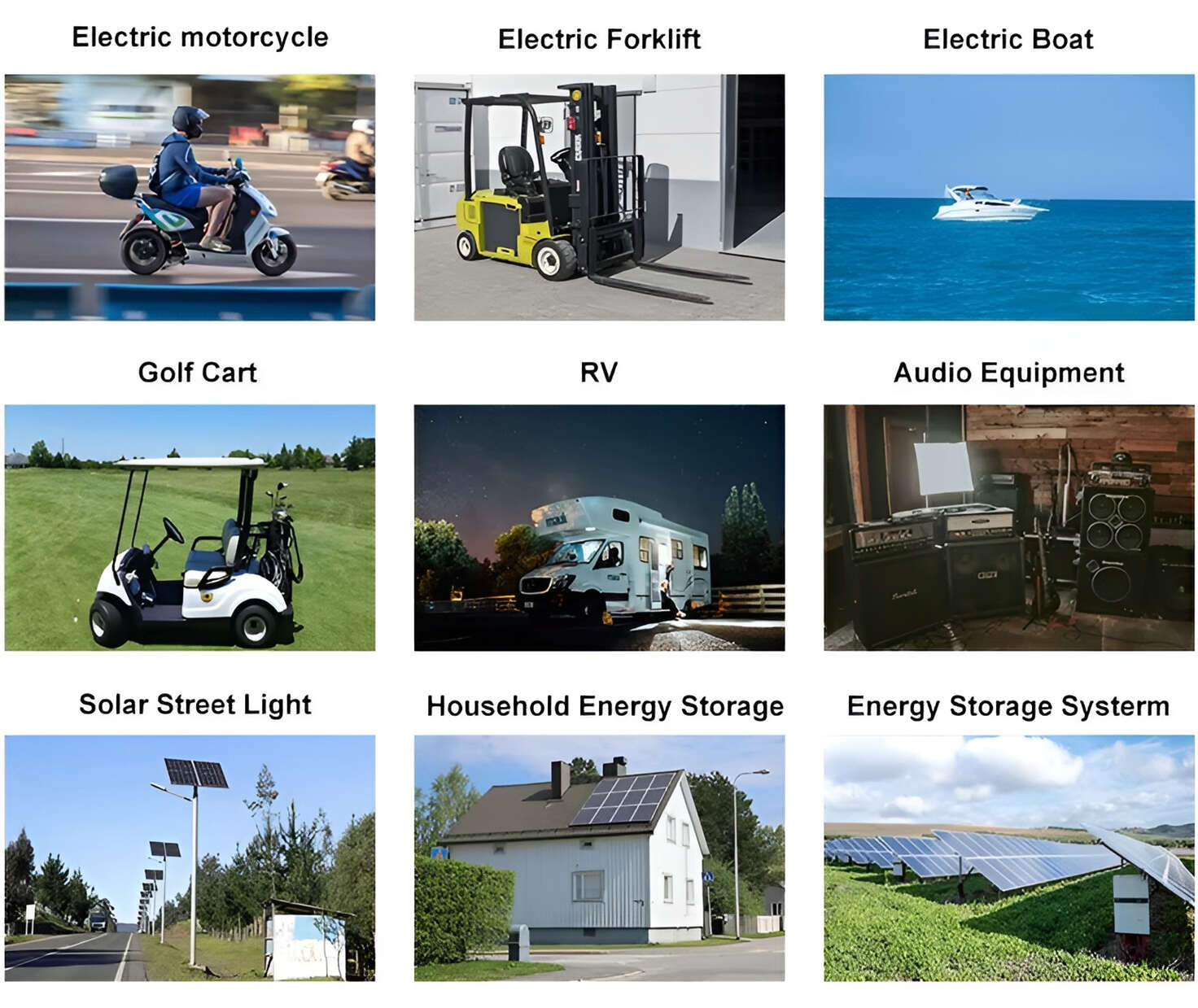
Applications in Different Industries
We supply customized & OEM battery pack, assemble cells with wiring, fuse and plastic cover, all the cell wires connected to PCB plug or built BMS.
Applications: E-bike, Electric Scooter, Golf Carts, RV, Electric Wheelchair, Electric Tools, Robot Cleaner, Robot Sweeper, Solar Energy Storage System, Emergency Light, Solar Power Light, Medical Equipment, UPS Backup Power Supply.
We can provide you with customized services. We have the ability to provide a vertical supply chain, from single cells to pack/module and to a complete power solution with BMS, etc.

HomSolar (Shenzhen) Technology Co., Ltd