The European Medicines Agency EMA has not 'admitted' that mRNA vaccines are 'experimental'
A false claim is circulating online that the European Medicines Agency (EMA) has acknowledged that mRNA vaccines have not been formally approved and that millions have therefore been vaccinated without clear guidelines.
Social posts refer to an article by a Swiss website called Uncut-News, which says that the EMA published a paper in January saying "there is no guideline which reflects the quality requirements for regulators and industry on mRNA containing vaccines".
Uncut-News says that while the paper focuses specifically on veterinary vaccines, this is still a worry for humans when these vaccines are administered on a large scale.
The website also takes issue with this line in the paper: "mRNA vaccines and their manufacturing process are a novel technology, and the resulting products differ from other types of vaccines".
Wilful misinterpretation and baseless criticismIt claims that this means that mRNA technology is experimental, giving credibility to anti-vaxxers, and goes on to suggest that millions were contaminated with unsafe, unregulated vaccines during the COVID-19 pandemic.

However, this is all misleading, as it misinterprets what the EMA document says and stirs up criticism of vaccines without any basis.
The cited paper is authentic and is explicit that it’s only dealing with mRNA vaccines for veterinary use.
It does reference COVID, but in a way that praises the regulation and testing of the vaccines used to combat the pandemic.
"In the area of human medicinal products, the number applications for clinical trials and marketing authorisations for mRNA containing products significantly increased over the last few years and a lot of experience with mRNA vaccines was gained during the COVID-19 pandemic," the EMA says in the paper.
It adds that this experience could be a great help in developing guidelines for the development of mRNA vaccines for veterinary use.
"Considering the scientific developments in recent years including the 29 experience gained during the COVID pandemic in the human medicinal products area and the first expected submissions for mRNA vaccines for veterinary use, such a guideline should be developed to ensure appropriate support in development and manufacturing of mRNA vaccines for veterinary use," the paper says.
'Novel' does not mean 'experimental'Respected public health bodies around the world assure that the mRNA COVID vaccines are safe.
The World Health Organization says that before receiving validation from regulatory agencies such as the EMA, "COVID-19 vaccines were subject to rigorous testing in clinical trials to prove that they meet internationally agreed benchmarks for safety and efficacy".
To give another example, John Hopkins Medicine in the US says that the vaccines "are very safe and very good at preventing serious or fatal cases of COVID-19".
"The risk of serious side effects associated with these vaccines is very small," it adds.
Another clue that the Uncut-News article is misleading is that it cites a post on X from an openly anti-vaxx account, with seemingly no expert credentials.
Related
Customized/OEM/ODM Service
HomSolar Supports Lifepo4 battery pack customization/OEM/ODM service, welcome to contact us and tell us your needs.


HomSolar: Your One-stop LiFePO4 Battery Pack & ESS Solution Manufacturer
Our line of LiFePO4 (LFP) batteries offer a solution to demanding applications that require a lighter weight, longer life, and higher capacity battery. Features include advanced battery management systems (BMS), Bluetooth® communication and active intelligent monitoring.

Customised Lithium Iron Phosphate Battery Casing
ABS plastic housing, aluminium housing, stainless steel housing and iron housing are available, and can also be designed and customised according to your needs.

HomSolar Smart BMS
Intelligent Battery Management System for HomSolar Energy Storage System. Bluetooth, temperature sensor, LCD display, CAN interface, UART interface also available.

Terminals & Plugs Can Be Customized
A wide range of terminals and plugs can be customised to suit the application needs of your battery products.

Well-designed Solutions for Energy Storage Systems
We will design the perfect energy storage system solution according to your needs, so that you can easily solve the specific industry applications of battery products.

About Our Battery Cells
Our energy storage system products use brand new grade A LiFePO4 cells with a battery lifespan of more than 4,000 charge/discharge cycles.

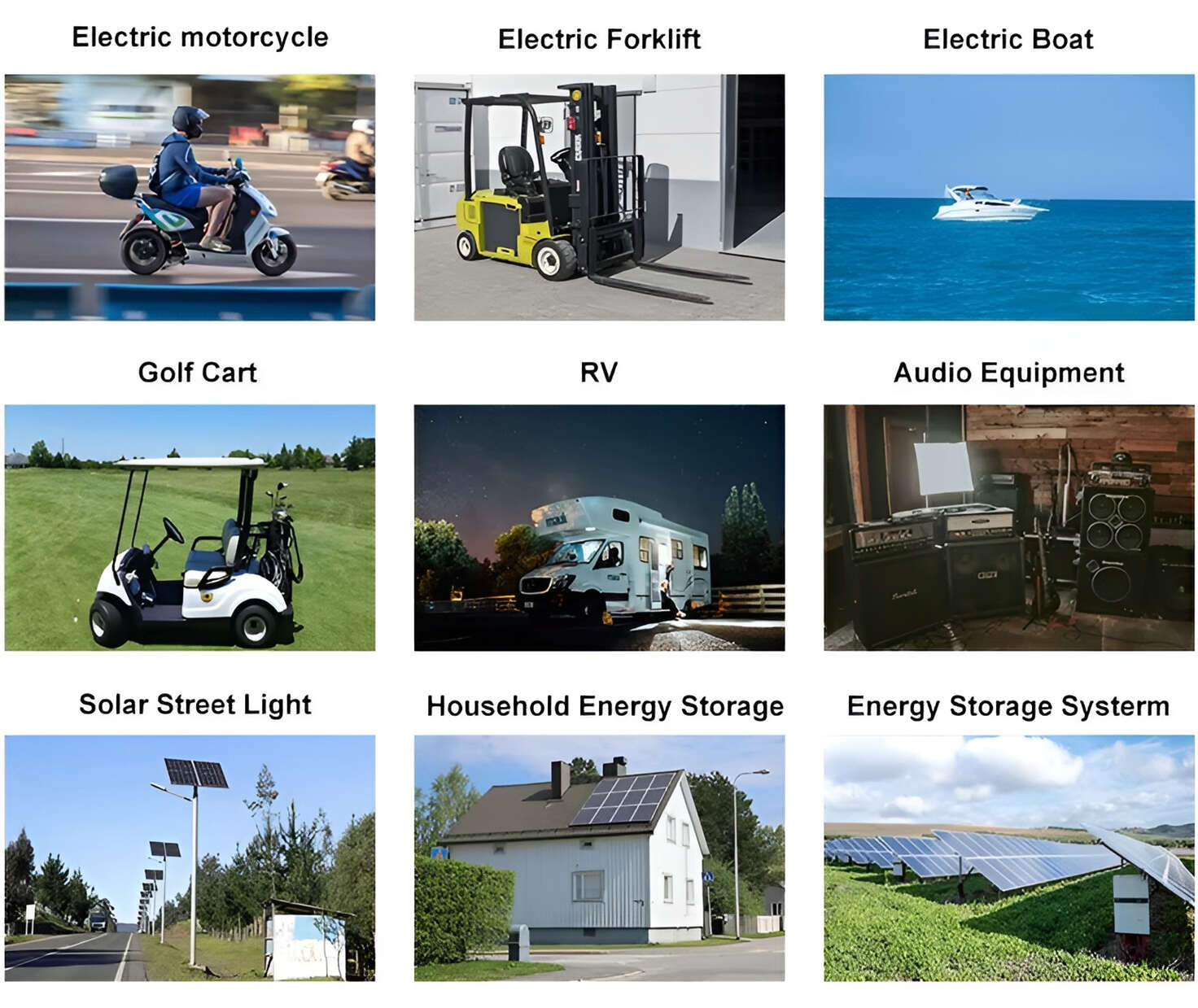
Applications in Different Industries
We supply customized & OEM battery pack, assemble cells with wiring, fuse and plastic cover, all the cell wires connected to PCB plug or built BMS.
Applications: E-bike, Electric Scooter, Golf Carts, RV, Electric Wheelchair, Electric Tools, Robot Cleaner, Robot Sweeper, Solar Energy Storage System, Emergency Light, Solar Power Light, Medical Equipment, UPS Backup Power Supply.
We can provide you with customized services. We have the ability to provide a vertical supply chain, from single cells to pack/module and to a complete power solution with BMS, etc.

HomSolar (Shenzhen) Technology Co., Ltd